Proposed law change to beef up penalties for drug violations

The penalties for companies that produce or sell fake or substandard pharmaceuticals in China are to be increased, according to a draft amendment to the country's Drug Management Law.
Manufacturers that operate without the required permit will face fines up to 30 times the value of their products, the draft states. Under current law, the maximum fine is five times the value.
The proposed amendment, now under review by the National People's Congress Standing Committee, the top legislature, states that violations such as making or selling fake drugs could see companies lose their business license.
Business executives and drug supervision officials will also face heavier punishment in cases involving pharmaceuticals, while supervision of certain drugs, such as vaccines, will be intensified to ensure safety and quality, it adds.
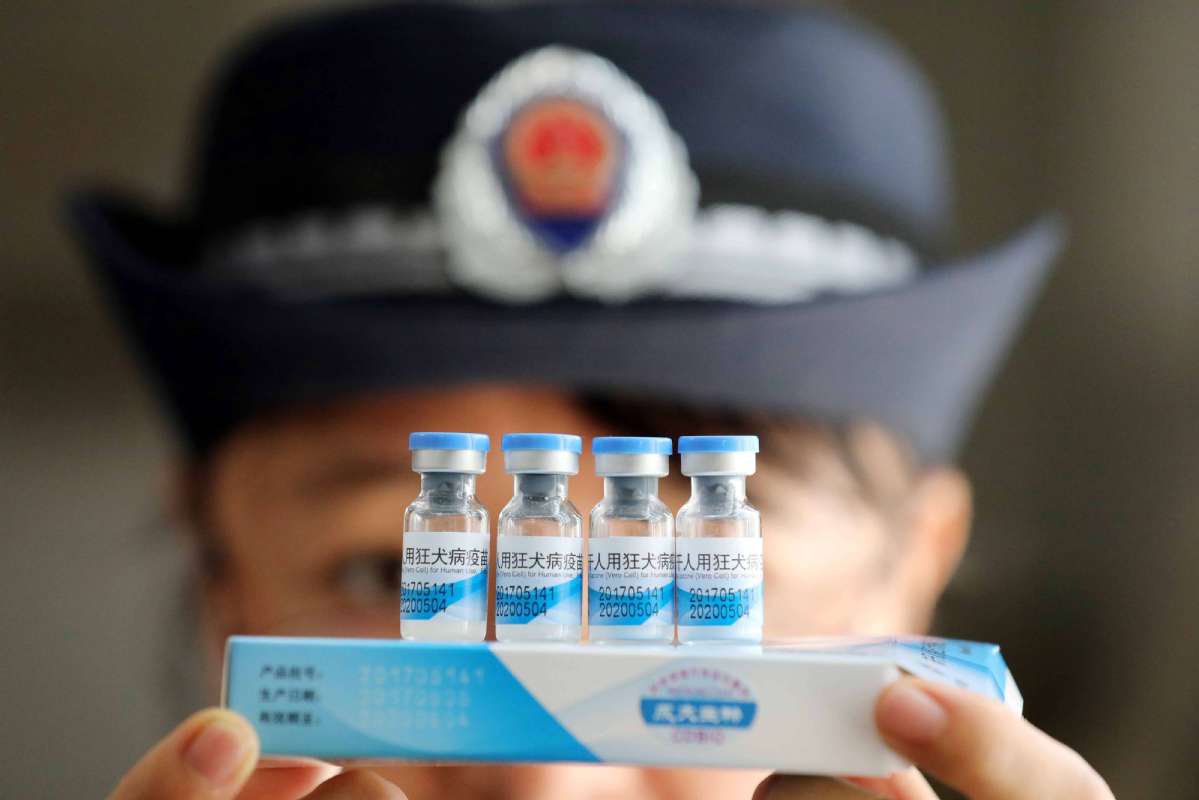
Jiao Hong, head of the National Medical Products Administration, said on Monday that the draft aims to improve supervision of drugs following the vaccine scandal at Changchun Changsheng Biotech, a major vaccine producer in Jilin province.
The administration fined the company 9.1 billion yuan ($1.3 billion) last week for fabricating production records and using expired materials to manufacture a human rabies vaccine.
- Domestic single-dose flu drug approved
- Water mahjong games cool visitors in Chongqing
- Sanya to host sixth Asian Beach Games in April
- Guangdong, Hong Kong and Macao to jointly hold entrepreneurship competition
- China approves rice-derived recombinant human albumin injection
- Hundreds attend 5th TCM Culture Festival in Tianjin